Difference between revisions of "Dynamics, threats and management of salt marshes"
| Line 15: | Line 15: | ||
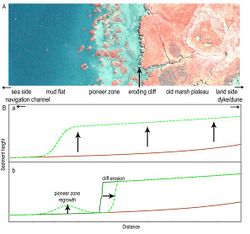
Many marshes are characterized by a cyclic nature, where marsh formation is followed by destruction (Figure 2). After a period of lateral extension, large scale lateral erosion of salt marshes can set in when the marsh edge becomes disturbed, a phenomenon often referred to as cliff erosion (see Figure 1.a, Figure 2.B.b; Allen 2000<ref name= "Allen">ALLEN J.R.L., 2000. Morphodynamics of Holocene salt marshes: a review sketch from the Atlantic and Southern North Sea coasts of Europe. ''Quaternary Science Reviews''. '''19''':, 1155-1231.</ref>; Adam 2002<ref name= "Adam">ADAM P., 2002. Salt marshes in a time of change. ''Environmental Conservation''. '''29''', 39‐61</ref>). For example, a disturbance from a storm surge can initialize this erosion process by forming a steep slope. At the disturbed edge, sediment is more vulnerable to wave action and currents. So once a cliff starts to erode, this process will not easily be stopped. Thus the steep slope remains particularly vulnerable for waves and currents until it is protected by new marsh vegetation emerging in front of the cliff. The initiation of cliff erosion is intrinsic to natural temporal salt marsh dynamics (Allen 2000<ref name= "Allen"/>; van de Koppel et al., 2005<ref>VAN DE KOPPEL J.,VAN DER WAL D., BAKKER J.P.,HERMAN P.M.J., 2005. Self‐Organization and Vegetation Collapse in Salt Marsh Ecosystems. ''The American Naturalist''. '''165''', E1-12.</ref>). However, human activities can contribute significantly to the severity of the cliff erosion (Allen 2000<ref name= "Allen"/>; Adam, 2002<ref name= "Adam"/>). For example, shipping traffic and dredging activities can increase exposure to currents and waves, thereby increasing the pace at which lateral erosion proceeds. Moreover, human induced activities may also take away the space for natural marsh recovery in front of the eroding cliff. The latter would result in the permanent loss of a marsh. | Many marshes are characterized by a cyclic nature, where marsh formation is followed by destruction (Figure 2). After a period of lateral extension, large scale lateral erosion of salt marshes can set in when the marsh edge becomes disturbed, a phenomenon often referred to as cliff erosion (see Figure 1.a, Figure 2.B.b; Allen 2000<ref name= "Allen">ALLEN J.R.L., 2000. Morphodynamics of Holocene salt marshes: a review sketch from the Atlantic and Southern North Sea coasts of Europe. ''Quaternary Science Reviews''. '''19''':, 1155-1231.</ref>; Adam 2002<ref name= "Adam">ADAM P., 2002. Salt marshes in a time of change. ''Environmental Conservation''. '''29''', 39‐61</ref>). For example, a disturbance from a storm surge can initialize this erosion process by forming a steep slope. At the disturbed edge, sediment is more vulnerable to wave action and currents. So once a cliff starts to erode, this process will not easily be stopped. Thus the steep slope remains particularly vulnerable for waves and currents until it is protected by new marsh vegetation emerging in front of the cliff. The initiation of cliff erosion is intrinsic to natural temporal salt marsh dynamics (Allen 2000<ref name= "Allen"/>; van de Koppel et al., 2005<ref>VAN DE KOPPEL J.,VAN DER WAL D., BAKKER J.P.,HERMAN P.M.J., 2005. Self‐Organization and Vegetation Collapse in Salt Marsh Ecosystems. ''The American Naturalist''. '''165''', E1-12.</ref>). However, human activities can contribute significantly to the severity of the cliff erosion (Allen 2000<ref name= "Allen"/>; Adam, 2002<ref name= "Adam"/>). For example, shipping traffic and dredging activities can increase exposure to currents and waves, thereby increasing the pace at which lateral erosion proceeds. Moreover, human induced activities may also take away the space for natural marsh recovery in front of the eroding cliff. The latter would result in the permanent loss of a marsh. | ||
| − | Loss of salt marsh habitat due to lateral erosion is a major problem across the world, especially in those locations where the marsh does not seem to recover. For example, the marshes in the Venice Lagoon (Italy) laterally erode with 1.2‐2.2 m yr-1at their seaward edges (Day ''et al.'', 1998<ref name= "Day">DAY J.W., SCARTON F., RISMONDO A., ARET D., 1998. Rapid Deterioration of a Salt Marsh in Venice Lagoon, Italy. ''Journal of Coastal Research''. '''14''', 583‐590.</ref>) .The estuaries of South‐East England lose about 4,000 m²yr-1 of tidal marsh area due to erosion at the seaward edges and channel widening of creeks dissecting the marsh (Hughes and Paramor, 2004<ref name= "Hughes">HUGHES R.G., PARAMOR O.A.L., 2004. On the loss of saltmarshes in south‐east England: methods for their restoration. '''Journal of Applied Ecology'''. '''41''': 440‐448.</ref>). However, the main drivers of salt marsh erosion are still subject of debate (Wolters ''et al.'', 2005<ref name= "Wolters">WOLTERS M., BAKKER J.P., BERTNESS M.D., JEFFERIES R.L., MÖLLER I., 2005. Saltmarsh erosion and restoration in south-east England: squeezing the evidence requires realignment. ''Journal of Applied Ecology''. '''42''', 844‐851. <ref | + | Loss of salt marsh habitat due to lateral erosion is a major problem across the world, especially in those locations where the marsh does not seem to recover. For example, the marshes in the Venice Lagoon (Italy) laterally erode with 1.2‐2.2 m yr-1at their seaward edges (Day ''et al.'', 1998<ref name= "Day">DAY J.W., SCARTON F., RISMONDO A., ARET D., 1998. Rapid Deterioration of a Salt Marsh in Venice Lagoon, Italy. ''Journal of Coastal Research''. '''14''', 583‐590.</ref>) .The estuaries of South‐East England lose about 4,000 m²yr-1 of tidal marsh area due to erosion at the seaward edges and channel widening of creeks dissecting the marsh (Hughes and Paramor, 2004<ref name= "Hughes">HUGHES R.G., PARAMOR O.A.L., 2004. On the loss of saltmarshes in south‐east England: methods for their restoration. '''Journal of Applied Ecology'''. '''41''': 440‐448.</ref>). However, the main drivers of salt marsh erosion are still subject of debate (Wolters ''et al.'', 2005<ref name= "Wolters">WOLTERS M., BAKKER J.P., BERTNESS M.D., JEFFERIES R.L., MÖLLER I., 2005. Saltmarsh erosion and restoration in south-east England: squeezing the evidence requires realignment. ''Journal of Applied Ecology''. '''42''', 844‐851. </ref>). Generally, it is believed that human activities are responsible for increasing erosion (Allen 2000<ref name= "Allen"/>; Adam 2002<ref name= "Adam"/>; Wolters ''et al.'', 2005<ref name= "Wolters"/>). Pollution, shipping and dredging are some of the proposed anthropogenic causes. In addition, climate change and sea level rise receivemuch attention as a cause of salt marsh disappearance. In addition to these extrinsic forcing factors, intrinsic biological processes are also proposed (Allen 2000<ref name= "Allen"/>; Wolters ''et al.'', 2005<ref name= "Wolters"/>). For example, vegetation‐sediment feedbacks (Allen 2000<ref name= "Allen"/>) and sediment destabilization by bioturbation and herbivory by worms (Hughes and Paramor, 2004<ref name= "Hughes"/>; van der Wal and Pye; 2004<ref name= "van der wal">VAN DER WAL D., PYE K., 2004. Patterns, rates and possible causes of saltmarsh erosion in the Greater Thames area (UK). ''Geomorphology''. '''61''', 373‐391.</ref>) and geese (Dionne, 1985<ref>DIONNE, J.-C., 1985. Tidal marsh erosion by Geese, St. Lawrence estuary, Québec. ''Géographie physique et Quaternaire''. '''39''', 99‐105.</ref>) can also result in erosion of salt marshes. A fundamental understanding of the mechanisms that control cliff initiation and salt marsh re‐establishment in front of a cliff is needed in order to protect and manage these highly dynamic salt marsh ecosystems. |
==VULNERABILITY & THREATS TO SALT MASRHES== | ==VULNERABILITY & THREATS TO SALT MASRHES== | ||
Revision as of 12:52, 16 July 2012
UNDER CONSTRUCTION
Contents
- 1 PPROCESSES AND MECHANISMS DRIVING NATURAL DYNAMICS & ECOSYSTEM DEVELOPMENT
- 2 VULNERABILITY & THREATS TO SALT MASRHES
- 3 KEY PROCESSES TO FOCUS ON FOR MAINTAINING ECOSYSTEMS INTEGRITY
- 4 CURRENT MANAGEMENT PRACTICES
- 5 see also
- 6 References

PPROCESSES AND MECHANISMS DRIVING NATURAL DYNAMICS & ECOSYSTEM DEVELOPMENT
Coastal areas, like estuaries, are high energetic environments where organisms are exposed to hydrodynamic forces from waves and tidal currents. Ecosystem engineering species (Jones et al., 1997) play an important role in shaping the intertidal landscape (Temmerman et al., 2007[1]; Weerman et al., 2010). Coastal vegetation, like salt marsh vegetation, are ecosystem engineers in that they can strongly attenuate hydrodynamic energy from tidal current and waves (Bouma et al., 2005[2], 2007[3], 2010). This has a positive effect on sediment accretion rates, and hence results in increased sediment elevation. In turn, increased sediment elevation stimulates plant growth because the inundation duration for the vegetation is shortened. This results in positive feedbacks between plant growth and sediment accretion. Implications of this feedback can be observed in the field in the form of dome shaped hummocks of cord‐grass (Spartina spp.). They can be found on the mud‐flats seaward of the salt marsh edge (Figure 1), where the salt marsh is developing.
Feedbacks between hydrodynamic forces, sediment accretion and vegetation are key processes in shaping salt marshes (Temmerman et al., 2007[1]; van Wesenbeeck et al., 2008). Locally the canopy of a vegetation stand can attenuate currents and waves which result in a net sedimentation. However, the same canopy also obstructs the flow, thereby diverting it and increasing flow velocities in the areas adjacent to the canopy because of conservation of mass and energy (Bouma et al., 2009). This biomechanical stress diversion can result in negative feedbacks on vegetation settlement and growth at some distance from the canopy (van Wesenbeeck et al., 2008). However, the outcome of these feedbacks may be dependent on the local context, seeing as these kinds of feedbacks are density‐dependent (Bouma et al., 2009). In other words, the strength of these negative feedbacks may vary with vegetation age, composition, or even the sediment type it is growing in (van Hulzen et al., 2006[4]). Overall these feedbacks cause complex patterns of gullies and hummocks until eventually a mature marsh arises, dissected by a complex drainage system (Kirwan and Murray, 2007; Temmerman et al., 2007[1]).
Many marshes are characterized by a cyclic nature, where marsh formation is followed by destruction (Figure 2). After a period of lateral extension, large scale lateral erosion of salt marshes can set in when the marsh edge becomes disturbed, a phenomenon often referred to as cliff erosion (see Figure 1.a, Figure 2.B.b; Allen 2000[5]; Adam 2002[6]). For example, a disturbance from a storm surge can initialize this erosion process by forming a steep slope. At the disturbed edge, sediment is more vulnerable to wave action and currents. So once a cliff starts to erode, this process will not easily be stopped. Thus the steep slope remains particularly vulnerable for waves and currents until it is protected by new marsh vegetation emerging in front of the cliff. The initiation of cliff erosion is intrinsic to natural temporal salt marsh dynamics (Allen 2000[5]; van de Koppel et al., 2005[7]). However, human activities can contribute significantly to the severity of the cliff erosion (Allen 2000[5]; Adam, 2002[6]). For example, shipping traffic and dredging activities can increase exposure to currents and waves, thereby increasing the pace at which lateral erosion proceeds. Moreover, human induced activities may also take away the space for natural marsh recovery in front of the eroding cliff. The latter would result in the permanent loss of a marsh.
Loss of salt marsh habitat due to lateral erosion is a major problem across the world, especially in those locations where the marsh does not seem to recover. For example, the marshes in the Venice Lagoon (Italy) laterally erode with 1.2‐2.2 m yr-1at their seaward edges (Day et al., 1998[8]) .The estuaries of South‐East England lose about 4,000 m²yr-1 of tidal marsh area due to erosion at the seaward edges and channel widening of creeks dissecting the marsh (Hughes and Paramor, 2004[9]). However, the main drivers of salt marsh erosion are still subject of debate (Wolters et al., 2005[10]). Generally, it is believed that human activities are responsible for increasing erosion (Allen 2000[5]; Adam 2002[6]; Wolters et al., 2005[10]). Pollution, shipping and dredging are some of the proposed anthropogenic causes. In addition, climate change and sea level rise receivemuch attention as a cause of salt marsh disappearance. In addition to these extrinsic forcing factors, intrinsic biological processes are also proposed (Allen 2000[5]; Wolters et al., 2005[10]). For example, vegetation‐sediment feedbacks (Allen 2000[5]) and sediment destabilization by bioturbation and herbivory by worms (Hughes and Paramor, 2004[9]; van der Wal and Pye; 2004[11]) and geese (Dionne, 1985[12]) can also result in erosion of salt marshes. A fundamental understanding of the mechanisms that control cliff initiation and salt marsh re‐establishment in front of a cliff is needed in order to protect and manage these highly dynamic salt marsh ecosystems.
VULNERABILITY & THREATS TO SALT MASRHES
Short-term effects of flooding and storms
SHORT-TERM FLOODING: VULNERABILITY OF MARSHES TO SALTWATER FLOODING
The salt‐marsh community is well adapted to salinity due to regular tidal exposure to seawater. The vast majority of salt-marshes are well drained and therefore at less risk to the endured flooding. In comparison, the community of grazing-marshes is adapted to very dilute seawater and the habitat drainage is often slow. The potential impact of saltwater flooding is therefore more severe for grazing marshes than for salt marshes. Much of the evidence regarding the effect of seawater on coastal vegetation therefore relates to oligohaline/grazing marshes.
SHORT-TERM FLOODING: Effect of flooding by saline water on salt marshes
A flooding event that originates from increased freshwater discharge, for instance due to heavy rainfall or ice melt in a catchment area, will result in a fresh‐water pulse through downstream marshes. Unless freshwater flooding lingers for extensive periods, the impact on the vegetation of salt-and grazing- marshes will be short lived (Flynn et al., 1995[13]; Grace and Ford, 1996Cite error: Closing </ref> missing for <ref> tag). The ‘halophytic’ (salt-tolerant) species that dominate salt-and grazing-marsh communities will not be harmed by short‐term fresh water exposure. However, their physiological and biochemical adaptations to cope with salinity stress make them poorly competitive under fresh water conditions (Crain et al., 2004[14]). Their halophytic traits enable them to colonise saline environments (Pennings and Callaway, 1992[15]). Salinity exposure in the salt marsh, and consequently the inherent salt tolerance of the inhabitant community, does not necessarily decline linearly with shore level. Summer evaporation of seawater pools can leave concentrated deposits of salt on the high marsh where the habitat is infrequently flushed by the, leading to high levels of sediment salinity that exclude less halophytic species (Watson and Burne, 2009). Paradoxically, increased frequency of seawater flushing by storms might dilute the accrued sediment salinity of such high marsh environments and alter the zonation of species. For instance, increased tidal flooding of an elevated marsh plain can cause the normally very halophytic high marsh species to be replaced by salt‐intolerant lower shore species (Watson and Burne, 2009).
The severity of impact of salt water flowing is likely to depend on the natural salinity occurring at a specific location. Relatively brackish-marshes, dominated by halophytic plants, will see less changes to community composition than fresh water dominated grazing marshes and coastal flood plains (Brown et al., 1994). Increased flooding by salt water is most likely to have the greatest effect on the fresh‐water adapted members of the marsh vegetation (Crain et al., 2004[14]), which increases in dominance in the transitional and grazing‐marsh above the tidal marks. Stormy conditions that result in a temporary increase in sea level and which bring in salt water pulses to coastal marsh systems therefore should have a greater effect on the grazing‐marsh community than on the salt marsh community. For example,seawater flooding of a diked grazing-marsh, following a dike breaching, prevented most of the fresh water vegetation from developing in the following spring (Klein and Bateman, 1998[16]). Vegetation cover, species richness, recovery and re-establishment of an oligohaline marsh decreased during one month of experimental exposures to increased salinity (from 0.5‐5.0 salinity up to 12) (Howard and Mendelssohn, 2000[17]). In the longer term, the space left by dead vegetation is likely to be colonised by more salinity tolerant species, and thus grazing-marsh communities might come to resemble those of salt marshes (Doody, 1982[18]; Howard and Mendelssohn, 2000[17]).
Many coastal marsh plants are able to recover temporary increases in salinity (Flynn et al., 1995[19]; Grace and Ford, 1996[20]; Howard and Mendelssohn, 2000[17]). However, the potential for lasting changes to communities increases with the duration of flooding (Flynn et al., 1995[19]; Howard and Mendelssohn 2000[17]). Elevated salinity (from natural, 0.5‐5 to 15) slowed vegetation recovery more in flooded than in drained soils (Flynn et al., 1995[19]). The naturally slow drainage of grazing marshes, that follows temporary sea water flood, causes this habitat to remain immersed for longer periods than salt marshes. Grazing marshes are therefore at greater risk to the endured flooding. However, there are indications that these marshes are relatively resilient to exposure; if the water is brackish enough, it may require months of immersion before significant impacts to vegetation cover occurs (e.g. Howard and Mendelssohn, 2000Cite error: Closing </ref> missing for <ref> tag proposed that an unexpected peak in vegetation species richness in the transitional marsh arose because pulsed variation in salinity (0‐5) prevented domination by fresh water or salt water species. Thus, pulsed salinity exposure might not necessarily diminish vegetation diversity. Nevertheless, increased salt water flooding of grazing marshes is likely to drive the succession towards more salt-tolerant vegetation, and increase the resemblance with salt marsh assemblages Note that the empirical evidence for the rate of this transition is lacking (Nicholls and Wilson, 2001[21]). The consequence of increased coastal flooding might therefore be a gradual loss of grazing-marsh communities, in exchange for gain in area cover of salt marsh communities (Doody, 1982[18]; Klein and Bateman 1998[16]; Nicholls and Wilson, 2001[21]).
SHORT-TERM FLOODING: Interactions of salinity with other disturbances
It is important to caution against a general interpretation that seawater flooding is a minimal risk to coastal marshes in general. The severity of seawater influence on grazing‐marshes might depend much on whether the salinity is paralleled with other plant stressors and disturbances. Sharpe and Balwin (2009)[22] sampled plant diversity in a marsh in the United States, across a fresh (salinity 0.5) to mesohaline (5-18) salinity gradient. In an undisturbed marsh, richness in transition zone oligohaline marshes was as high as or higher than in tidal fresh water-marshesIn an anthropogenically disturbed estuary, however, plant species richness declined linearly with an increase in salinity. Experimental flooding by brackish (6‐14) water had a greater effect on grazing‐marsh community structure and biomass when the vegetation was also disturbed by leaf clipping (Baldwin and Mendelssohn, 1998) or grazing (Gough and Grace, 1998). In comparison, flooding did not affect species richness in the absence of such additional disturbances (Baldwin and Mendelssohn, 1998). If the salinity and water regimes are permanently altered and/or the vegetation is destroyed by a combination of factors, the substrate might eventually subside. Substrate subsidence and associated increased water depth might prevent seed dispersal and germination of more flooding tolerant species, and thus hamper system recovery (McKee and Mendelssohn, 1989[23]).It is not known beyond which threshold the frequency of flooding will have permanent effects.
Long-term effects due to climate change and sea level rise
KEY PROCESSES TO FOCUS ON FOR MAINTAINING ECOSYSTEMS INTEGRITY
CURRENT MANAGEMENT PRACTICES
see also
References
- ↑ 1.0 1.1 1.2 TEMMERMAN, S.; BOUMA, T.J.; VAN DE KOPPEL, J.; VAN DER WAL, D.; DE VRIES, M.B.; HERMAN, P.M.J.(2007). Vegetation causes channel erosion in a tidal landscape. Geology. 35(7), 631-634. Available from: http://www.vliz.be/imis/imis.php?module=ref&refid=114118
- ↑ BOUMAT.J.,DE VRIES M.B., LOW E., PERALTA G., TNCZOSI.C.,VANDEKOPPELJ., HERMAN P. M. J., 2005. Trade‐offs Related to Ecosystem Engineering: A Case Study on Stiffness of Emerging Macrophytes. Ecology. 86, 2187‐2199.
- ↑ BOUMA, T.J.; VAN DUREN, L.A.; TEMMERMAN, S.; CLAVERIE, T.; BLANCO-GARCIA, A.; YSEBAERT, T.J.; HERMAN, P.M.J. (2007). Spatial flow and sedimentation patterns within patches of epibenthic structures. Cont. Shelf Res.. 27(8): 1020-1045. dx.doi.org/10.1016/j.csr.2005.12.019 Available from:http://www.vliz.be/imis/imis.php?module=ref&refid=114437
- ↑ VAN HULZEN J.,VAN SOELEN J.,HERMAN P.M.J., BOUMA T.J., 2006.The significance of spatial andtemporal patterns of algal mat deposition in structuring salt marsh vegetation. J Veget Sci.. 17, 291‐298.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 ALLEN J.R.L., 2000. Morphodynamics of Holocene salt marshes: a review sketch from the Atlantic and Southern North Sea coasts of Europe. Quaternary Science Reviews. 19:, 1155-1231.
- ↑ 6.0 6.1 6.2 ADAM P., 2002. Salt marshes in a time of change. Environmental Conservation. 29, 39‐61
- ↑ VAN DE KOPPEL J.,VAN DER WAL D., BAKKER J.P.,HERMAN P.M.J., 2005. Self‐Organization and Vegetation Collapse in Salt Marsh Ecosystems. The American Naturalist. 165, E1-12.
- ↑ DAY J.W., SCARTON F., RISMONDO A., ARET D., 1998. Rapid Deterioration of a Salt Marsh in Venice Lagoon, Italy. Journal of Coastal Research. 14, 583‐590.
- ↑ 9.0 9.1 HUGHES R.G., PARAMOR O.A.L., 2004. On the loss of saltmarshes in south‐east England: methods for their restoration. Journal of Applied Ecology. 41: 440‐448.
- ↑ 10.0 10.1 10.2 WOLTERS M., BAKKER J.P., BERTNESS M.D., JEFFERIES R.L., MÖLLER I., 2005. Saltmarsh erosion and restoration in south-east England: squeezing the evidence requires realignment. Journal of Applied Ecology. 42, 844‐851.
- ↑ VAN DER WAL D., PYE K., 2004. Patterns, rates and possible causes of saltmarsh erosion in the Greater Thames area (UK). Geomorphology. 61, 373‐391.
- ↑ DIONNE, J.-C., 1985. Tidal marsh erosion by Geese, St. Lawrence estuary, Québec. Géographie physique et Quaternaire. 39, 99‐105.
- ↑ FLYNN, K.M.; MCKEE, .KL.; MENDELSSOHN, I.A, 1995.RECOVERY OF FRESH-WATER MARSH VEGETATION AFTER A SALTWATER INTRUSION EVENT. OECOLOGIA. 103(1), 63-72 DOI:10.1007/BF00328426.
- ↑ 14.0 14.1 CRAIN, C.M.; SILLIMAN B.R.; BERTNESS, S.L. ; BERTNESS, M.D., 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients. ECOLOGY. 85(9), 2539-2549.DOI: 10.1890/03-0745
- ↑ CRAIN, C.M.; SILLIMAN B.R.; BERTNESS, S.L. ; BERTNESS, M.D., 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients. ECOLOGY. 85(9), 2539-2549. DOI: 10.1890/03-0745
- ↑ 16.0 16.1 KLEIN R.J.T., BATEMAN I.J., 1998. The recreational value of Cley marshes nature reserve: An argument against managed Retreat? Water and Environment Journal. 12, 280-285.
- ↑ 17.0 17.1 17.2 17.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedHoward - ↑ 18.0 18.1 DOODY J.P., 1982. Sea defence and nature conservation: threat or opportunity. Aquat Conserv Mar Freshw Ecosyst, 2, 275-283.
- ↑ 19.0 19.1 19.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedFlynn - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedGrace - ↑ 21.0 21.1 NICHOLLS R.J., WILSON T., 2001. Chapter five. Integrated impacts on coastal areas and river flooding. In: Holman I.P., Loveland P.J. (Eds), Regional Climate Change Impact and Response Studies in East Anglia and North West England (RegIS). Final Report of MAFF project no. CC0337. (downloadable at www.ukcip.org.uk).
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedSharpe - ↑ MCKEE K.L.; MENDELSSOHN I.A.,1989. Response of a fresh-water marsh plant community to increased salinity and increased water level. AQUATIC BOTANY, 34(4): 301-316. DOI: 10.1016/0304-3770(89)90074-0.
Please note that others may also have edited the contents of this article.
|
