| Intro | | About | | Wiki | | Search traits | | Data explorer | | Literature | | Definitions | | Sources | | Webservices | | Statistics | | Feedback | | Editors | | Log in |
WoRMS taxon detailsAlexandrium catenella (Whedon & Kofoid) Balech, 1985
231873 (urn:lsid:marinespecies.org:taxname:231873)
accepted
Species
Alexandrium excavatum (Braarud) Balech & Tangen, 1985 · unaccepted (synonym)
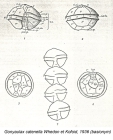
Alexandrium fundyense Balech, 1985 · unaccepted (name rejected; "catenella" has...)
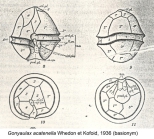
name rejected; "catenella" has nomenclatural priority Gonyaulax acatenella Whedon & Kofoid, 1936 · unaccepted (basionym)
Gonyaulax catenella Whedon & Kofoid, 1936 · unaccepted (basionym)
Gonyaulax excavata (Braarud) Balech, 1971 · unaccepted (synonym)
Gonyaulax tamarensis var. excavata T.Braarud, 1945 · unaccepted (synonym)
Gonyaulax washingtonensis Hsu, 1967 · unaccepted (synonym)
Protogonyaulax excavata (Braarud) F.J.R.Taylor, 1979 · unaccepted (synonym)
marine,
(of Gonyaulax catenella Whedon & Kofoid, 1936) Whedon W.F. & Kofoid C.A. 1936. Dinoflagellates of the San Francisco region. I. On the skeletal morphology of two new species, <i>Gonyaulax catenella</i> and <i>G. acatenella</i>. Univ. Calif. Publ. Zool. 41: 25-34. [details] Available for editors
Type locality contained in San Francisco
type locality contained in San Francisco [details]
LSID urn:lsid:algaebase.org:taxname:52031
LSID urn:lsid:algaebase.org:taxname:52031 [details] Description Resting of A. catenella are elongate-cylindrical cells with rounded ends. Length varied from 43-72 µm and width from 26-39...
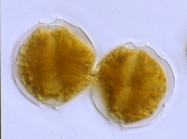
Description Resting of A. catenella are elongate-cylindrical cells with rounded ends. Length varied from 43-72 µm and width from 26-39 µm. The multilayered wall is thick (2-5 µm). Hypnocysts of A. catenella and A. tamarense are identical under the light microscope. [details] Description Cell is small- to medium-sized, somewhat flattened anterior-posteriorly. This species usually forms curved chains. Epitheca...
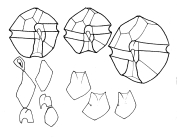
Description Cell is small- to medium-sized, somewhat flattened anterior-posteriorly. This species usually forms curved chains. Epitheca has more or less noticeable shoulders and a rather upraised apical region. Cingulum is very excavated, descending (1, sometimes a little more). It generally has an overlapping membrane or curtain fin that extends from the projecting flange on the epitheca to the corresponding flange on the hypotheca. Sulcus is rather deep, abruptly widened on the posterior. Sulcal membranes are moderate, but sometimes they project slightly toward the posterior region and look like one or two small spines. On some occasions, the antapical left lobule is a little longer and rounder than the right one. PO is wide with a dorsal margin that is oblique and almost straight. The left margin is regular or irregularly convex. The right one is sinuous, almost always straight or slightly convex in its dorsal half and concave in its ventral half. The ventral portion of the plate is usually clearly and obliquely truncated, which provides extensive contact with 1'. The comma has a large head that is close to the left margin and has a thick callus. The canopy is narrow and translucent. A connecting pore that is medium-sized and elliptically shaped is located near the comma head to its right. Frequently, accessory pores are present, especially a more ventral one. 1' does not have a ventral pore and directly contacts the PO. It is asymmetrically rhomboidal (each pair of long and short sides is parallel). Usually, the anterior right margin is clearly concave. The anterior and posterior corners are more or less extensively truncated. Plates 2', 3', and 4' have raised flanges that support the PO and are frequently denticulated, especially 2'. 2' is the largest apical plate and is usually connected with 3' by a sinuous margin that is mostly concave. 3' is hexagonal and clearly asymmetrical with the anterior left side up to twice as long as the anterior right side. 4' is relatively short and wide. In the precingular series, only 6"" is noteworthy. It is medium wide. Its internal margin borders with the S.a. and has a barely pronounced concavity. In the hypotheca, 5""' is wide and has a. somewhat reinforced internal margin that is slightly E-shaped and supports a narrow iist. Its anterior-internal corner is usually briefly truncated and reinforced. 1"""" is rather narrow: Iong, and very oblique. Its sulcal list is moderately wide, wider anteriorly than posterioriy. 2'""' is transversely elongated (type B). In the sulcus, the S.a. is longer than wide or as long as wide. It does not extend above the anterior-left margin of the cingulum. A deep posterior sinus is present. The anterior margin is wide and almost stiaight. A fold may be obliquely directed from the left anterior to the right posterior of the plate, usually ending before it reaches the notch. The S.p. is asymmetric, rhombic, and almost as wide as long. The margin that borders S.s.p. and S.d.p. is scooped. The margas that border the 1"""" and 5""' are almost straight. Where they join with the curved ventral margin, two crests are formed. These crests are directedposteriorly then diminish before reaching the plate midpoint. The two dorsal margins are usually rather convex and are distinct 5om each other because the left one is longer. A large, irregulariy shaped connecting pore is located a little to the right of plate center. The pore is dorsoventrally elongated and either quadrangular or triangular with more or less obtuse vertices. It is linked by a small charnel to the ventra! right margin. The S.s.a. deflects the left sulcal margin outward in a rather pronounced way. It is relatively wide, rather shallowly set in the sulcus, and irregularly oval-shaped. Its two posterior margins form a rather obvious angle. The S.s.p. is trapezoid-shaped. Its anterior left margin adjoins the S.s.a. and is rather long and very oblique. The anterior internal corner is truncated, which forms a short an [details] Distribution Alexandrium catenella is present in the North Pacific coasts from North Japan in the West to California in the West coast...
Distribution Alexandrium catenella is present in the North Pacific coasts from North Japan in the West to California in the West coast of North America. In the North Atlantic, from Cape Cod in the USA to the British Islands including the North Sea. In the South Pacific in South Chile and Tasmania. In the South Atlantic, in the Southern Argentina and SW Africa. [details]
Guiry, M.D. & Guiry, G.M. (2024). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway (taxonomic information republished from AlgaeBase with permission of M.D. Guiry). Alexandrium catenella (Whedon & Kofoid) Balech, 1985. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=231873 on 2024-11-10
Date action by 2006-07-27 06:59:07Z changed Camba Reu, Cibran Copyright notice: the information originating from AlgaeBase may not be downloaded or replicated by any means, without the written permission of the copyright owner (generally AlgaeBase). Fair usage of data in scientific publications is permitted.
original description
(of Alexandrium fundyense Balech, 1985) Balech, E. (1985). The genus <i>Alexandrium</i> or <i>Gonyaulax</i> of the <i>tamarensis</i> group. In: D.M. Anderson, A.W. White and D.G. Baden (Eds.) Toxic Dinoflagellates. New York: Elsevier. P. 33–38. [details] Available for editors
original description (of Alexandrium acatenella (Whedon & Kofoid) Balech, 1985) Whedon W.F. & Kofoid C.A. 1936. Dinoflagellates of the San Francisco region. I. On the skeletal morphology of two new species, <i>Gonyaulax catenella</i> and <i>G. acatenella</i>. Univ. Calif. Publ. Zool. 41: 25-34. [details] Available for editors original description (of Gonyaulax catenella Whedon & Kofoid, 1936) Whedon W.F. & Kofoid C.A. 1936. Dinoflagellates of the San Francisco region. I. On the skeletal morphology of two new species, <i>Gonyaulax catenella</i> and <i>G. acatenella</i>. Univ. Calif. Publ. Zool. 41: 25-34. [details] Available for editors basis of record Gómez, F. (2005). A list of free-living dinoflagellate species in the world's oceans. <em>Acta Bot. Croat.</em> 64(1): 129-212. [details] additional source Guiry, M.D. & Guiry, G.M. (2024). AlgaeBase. <em>World-wide electronic publication, National University of Ireland, Galway.</em> searched on YYYY-MM-DD., available online at http://www.algaebase.org [details] additional source Tomas, C.R. (Ed.). (1997). Identifying marine phytoplankton. Academic Press: San Diego, CA [etc.] (USA). ISBN 0-12-693018-X. XV, 858 pp., available online at http://www.sciencedirect.com/science/book/9780126930184 [details] additional source Miranda, L. N.; Zhuang, Y.; Zhang, H.; Lin, S. (2012). Phylogenetic analysis guided by intragenomic SSU rDNA polymorphism refines classification of “<i>Alexandrium tamarense</i>” species complex. <em>Harmful Algae.</em> 16: 35-48., available online at https://doi.org/10.1016/j.hal.2012.01.002 [details] additional source Wang, L.; Zhuang, Y.; Zhang, H.; Lin, X.; Lin, S. (2014). DNA barcoding species in Alexandrium tamarense complex using ITS and proposing designation of five species. <em>Harmful Algae.</em> 31: 100-113., available online at https://doi.org/10.1016/j.hal.2013.10.013 [details] additional source Liu, J.Y. [Ruiyu] (ed.). (2008). Checklist of marine biota of China seas. <em>China Science Press.</em> 1267 pp. (look up in IMIS) [details] Available for editors additional source Chang, F.H.; Charleston, W.A.G.; McKenna, P.B.; Clowes, C.D.; Wilson, G.J.; Broady, P.A. (2012). Phylum Myzozoa: dinoflagellates, perkinsids, ellobiopsids, sporozoans, in: Gordon, D.P. (Ed.) (2012). New Zealand inventory of biodiversity: 3. Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi. pp. 175-216. [details] additional source Lutaenko, K.A.; Furota, T.; Nakayama; S.; Shin, K.; Xu, J. (2013). Atlas of Marine Invasive Species in the NOWPAP Region. Beijing: NOWPAP DINRAC (Northwest Pacific Action Plan, Data and Information Network Regional Center). 189 pp. [details] additional source Balech, E. (2002). Dinoflagelados tecados tóxicos en el Cono Sur Americano. <em>In: Sar, E.A., Ferrario, M.E. & Reguera, B. (Eds.). Floraciones Algales Nocivas en el Cono Sur Americano. Instituto Español de Oceanografía.</em> pp. 123-144. [details] Available for editors additional source Prud'homme van Reine, Willem F. (2017). Report of the Nomenclature Committee for Algae: 15. <em>Taxon.</em> 66(1): 191–192; February 2017., available online at https://doi.org/10.12705/661.16 [details] Available for editors additional source Zenetos, A.; Çinar, M.E.; Pancucci-Papadopoulou, M.A.; Harmelin, J.-G.; Furnari, G.; Andaloro, F.; Bellou, N.; Streftaris, N.; Zibrowius, H. (2005). Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. <em>Mediterranean Marine Science.</em> 6 (2): 63-118., available online at https://www.researchgate.net/publication/273213810_Annotated_list_of_marine_alien_species_in_the_Mediterranean_with_records_of_the_worst_invasive_species [details] Available for editors new combination reference Balech, E. (1985). The genus <i>Alexandrium</i> or <i>Gonyaulax</i> of the <i>tamarensis</i> group. In: D.M. Anderson, A.W. White and D.G. Baden (Eds.) Toxic Dinoflagellates. New York: Elsevier. P. 33–38. [details] Available for editors toxicology source Burke J.M., Marchisotto J., McLaughlin J.J.A. & Provasoli L. 1960. Analysis of the toxin produced by <i>Gonyaulax catenella</i> in axenic culture. Ann. N. Y. Acad. Sci. 90: 837-842. [details] toxicology source Bates H.A., Kostriken R. & Rapoport H. 1978. The occurrence of saxitoxin and other toxins in various dinoflagellates. Toxicon 16: 595-601. [details] toxicology source Aguilera-Belmonte, A.; Inostroza, I.; Franco, J. M.; Riobó, P.; Gómez, P. I. (2011). The growth, toxicity and genetic characterization of seven strains of <i>Alexandrium catenella</i> (Whedon and Kofoid) Balech 1985 (Dinophyceae) isolated during the 2009 summer outbreak in southern Chile. <em>Harmful Algae.</em> 12: 105-112., available online at https://doi.org/10.1016/j.hal.2011.09.006 [details] ecology source Jeong, H.; Park, J.; Nho, J.; Park, M.; Ha, J.; Seong, K.; Jeng, C.; Seong, C.; Lee, K.; Yih, W. (2005). Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. <em>Aquatic Microbial Ecology.</em> 41: 131-143., available online at https://doi.org/10.3354/ame041131 [details] ecology source Nygaard, K.; Tobiesen, A. (1993). Bacterivory in algae: A survival strategy during nutrient limitation. <em>Limnology and Oceanography.</em> 38(2): 273-279., available online at https://doi.org/10.4319/lo.1993.38.2.0273 [details] ecology source Du Yoo, Y.; Jeong, H. J.; Kim, M. S.; Kang, N. S.; Song, J. Y.; Shin, W.; Kim, K. Y.; Lee, K. (2009). Feeding by Phototrophic Red-Tide Dinoflagellates on the Ubiquitous Marine Diatom<i>Skeletonema costatum</i>. <em>Journal of Eukaryotic Microbiology.</em> 56(5): 413-420., available online at https://doi.org/10.1111/j.1550-7408.2009.00421.x [details] ecology source Jeong, H.; Yoo, Y.; Park, J.; Song, J.; Kim, S.; Lee, S.; Kim, K.; Yih, W. (2005). Feeding by phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. <em>Aquatic Microbial Ecology.</em> 40: 133-150., available online at https://doi.org/10.3354/ame040133 [details] ecology source Leles, S. G.; Mitra, A.; Flynn, K. J.; Tillmann, U.; Stoecker, D.; Jeong, H. J.; Burkholder, J.; Hansen, P. J.; Caron, D. A.; Glibert, P. M.; Hallegraeff, G.; Raven, J. A.; Sanders, R. W.; Zubkov, M. (2019). Sampling bias misrepresents the biogeographical significance of constitutive mixotrophs across global oceans. <em>Global Ecology and Biogeography.</em> 28(4): 418-428., available online at https://doi.org/10.1111/geb.12853 [details] Available for editors ecology source Mitra, A.; Caron, D. A.; Faure, E.; Flynn, K. J.; Leles, S. G.; Hansen, P. J.; McManus, G. B.; Not, F.; Do Rosario Gomes, H.; Santoferrara, L. F.; Stoecker, D. K.; Tillmann, U. (2023). The Mixoplankton Database (MDB): Diversity of photo‐phago‐trophic plankton in form, function, and distribution across the global ocean. <em>Journal of Eukaryotic Microbiology.</em> 70(4)., available online at https://doi.org/10.1111/jeu.12972 [details]  Present Present  Present in aphia/obis/gbif/idigbio Present in aphia/obis/gbif/idigbio  Inaccurate Inaccurate  Introduced: alien Introduced: alien  Containing type locality Containing type locality
From editor or global species database
LSID urn:lsid:algaebase.org:taxname:52031 [details]From regional or thematic species database
Description Resting of A. catenella are elongate-cylindrical cells with rounded ends. Length varied from 43-72 µm and width from 26-39 µm. The multilayered wall is thick (2-5 µm). Hypnocysts of A. catenella and A. tamarense are identical under the light microscope. [details] Description Cell is small- to medium-sized, somewhat flattened anterior-posteriorly. This species usually forms curved chains. Epitheca has more or less noticeable shoulders and a rather upraised apical region. Cingulum is very excavated, descending (1, sometimes a little more). It generally has an overlapping membrane or curtain fin that extends from the projecting flange on the epitheca to the corresponding flange on the hypotheca. Sulcus is rather deep, abruptly widened on the posterior. Sulcal membranes are moderate, but sometimes they project slightly toward the posterior region and look like one or two small spines. On some occasions, the antapical left lobule is a little longer and rounder than the right one. PO is wide with a dorsal margin that is oblique and almost straight. The left margin is regular or irregularly convex. The right one is sinuous, almost always straight or slightly convex in its dorsal half and concave in its ventral half. The ventral portion of the plate is usually clearly and obliquely truncated, which provides extensive contact with 1'. The comma has a large head that is close to the left margin and has a thick callus. The canopy is narrow and translucent. A connecting pore that is medium-sized and elliptically shaped is located near the comma head to its right. Frequently, accessory pores are present, especially a more ventral one. 1' does not have a ventral pore and directly contacts the PO. It is asymmetrically rhomboidal (each pair of long and short sides is parallel). Usually, the anterior right margin is clearly concave. The anterior and posterior corners are more or less extensively truncated. Plates 2', 3', and 4' have raised flanges that support the PO and are frequently denticulated, especially 2'. 2' is the largest apical plate and is usually connected with 3' by a sinuous margin that is mostly concave. 3' is hexagonal and clearly asymmetrical with the anterior left side up to twice as long as the anterior right side. 4' is relatively short and wide. In the precingular series, only 6"" is noteworthy. It is medium wide. Its internal margin borders with the S.a. and has a barely pronounced concavity. In the hypotheca, 5""' is wide and has a. somewhat reinforced internal margin that is slightly E-shaped and supports a narrow iist. Its anterior-internal corner is usually briefly truncated and reinforced. 1"""" is rather narrow: Iong, and very oblique. Its sulcal list is moderately wide, wider anteriorly than posterioriy. 2'""' is transversely elongated (type B). In the sulcus, the S.a. is longer than wide or as long as wide. It does not extend above the anterior-left margin of the cingulum. A deep posterior sinus is present. The anterior margin is wide and almost stiaight. A fold may be obliquely directed from the left anterior to the right posterior of the plate, usually ending before it reaches the notch. The S.p. is asymmetric, rhombic, and almost as wide as long. The margin that borders S.s.p. and S.d.p. is scooped. The margas that border the 1"""" and 5""' are almost straight. Where they join with the curved ventral margin, two crests are formed. These crests are directedposteriorly then diminish before reaching the plate midpoint. The two dorsal margins are usually rather convex and are distinct 5om each other because the left one is longer. A large, irregulariy shaped connecting pore is located a little to the right of plate center. The pore is dorsoventrally elongated and either quadrangular or triangular with more or less obtuse vertices. It is linked by a small charnel to the ventra! right margin. The S.s.a. deflects the left sulcal margin outward in a rather pronounced way. It is relatively wide, rather shallowly set in the sulcus, and irregularly oval-shaped. Its two posterior margins form a rather obvious angle. The S.s.p. is trapezoid-shaped. Its anterior left margin adjoins the S.s.a. and is rather long and very oblique. The anterior internal corner is truncated, which forms a short an [details] Distribution Alexandrium catenella is present in the North Pacific coasts from North Japan in the West to California in the West coast of North America. In the North Atlantic, from Cape Cod in the USA to the British Islands including the North Sea. In the South Pacific in South Chile and Tasmania. In the South Atlantic, in the Southern Argentina and SW Africa. [details] Harmful effect Producer of paralytic shellfish poisoning toxins and fish mass mortality causative substance. Alexandrium catenella may cause severe PSP events and is a common cause of human deaths in cold temperate coastal areas (i.e. strain PFB39 in Aguilera-Belmonte et al. 2011). Although it can cause toxicity at low cells concentrations, big blooms are not rare events. It produces a series of toxins of the saxitoxin group having different toxin profiles. [details] Identification Epifluorescence light microscopy of cells stained with calcofluor is the best method for identification of Alexandrium catenella. Nevertheless, due to the great morphological variability of this species, i.e. Presence or absence of ventral pore, or chain formation, it is difficult or impossible to distinguish it from closely related species without a previous knowledge of the local flora. Molecular techniques are usually needed for a proper identification. [details] Identification This species shares with A. kutnerae and A. cohorticula, a very distinctive character: the S.a. has a well-developed precingular region bordered posteriorly by a transversal bar or rib. However, A. kutnerae is much larger and has a very different globose shape. In A. kutnerae, 1' is narrower and frequently is connected with the PO indirectly. Its ventral pore is almost always enclosed within the 1' plate and, sometimes, is even displaced inward and separated from the margin. The 1"""" of A. kutnerae is wider and more regular. 2"""" is more regular and not clearly transversely elongated. It does not form chains. Its S.p. is narrower, almost never with a connecting pore. The S.d.a is approximately as long as it is wide. The most similar species to A. tamiyavanichi is A. cohorticula, so much so, that it was presented as A. cohorticula by Japanese researchers (see synonymy). Both are chain formers and their sizes are equivalent. However, A. cohorticula has a more irregular shape and an epitheca that is longer than wide. Its PO is comparably longer and more sharp-pointed. The comma is not central but is somewhat displaced ventrally and, therefore, leaves a wider dorsal margin. 1' is narrower and more irregular and has a straight and more or less horizontal posterior margin that contrasts with the curvature and large obliquity of the corresponding margin in A. tamiyavanichi. The left sulcal list of A. cohorticula is exceptionally wide anteriorly and it abruptly lessens posteriorly, The S.p. has a connecting pore that is larger and oval. The S.a. of A. cohorticula has a precingular portion that is longer and square, limited posteriorly by a very strong and straight or almost straight bar. Its S.s.a. is longer and has a different shape. Its S.d.a, is not wider than long. Its S.d.p. is generally more robust and it does not have such an oblique anterior margin. S.d.a., in the S.a., and in the list of 1"""" seem to offer valid characters for the specific separation of this species. It can be supposed, however, that some other cited differences are phenotypical. [details] Identification Based on studies of ribosomal genes, species of the so-called tamarense group fall into 5 groups. It has been suggested that each group should be considered a separate species. Group I contains several morphospecies, including A. catenella, A. tamarense and A. fundyense (John et al. 2014). It has been suggested that Group I should be named A. fundyense (John et al 2014). If the ribospecies concept is accepted for the tamarense group, it has serious implications for the naming of many isolates, and studies on additional genes are needed to confirm or refute the above suggestion. Material from the type locality of A. tamarense is not part of Group I. However, both the type localities of A. catenella and A. fundyense fall into the geographical distribution of Group I. It has been proposed formally to the Nomenclature Committee for Algae (John et al. 2014) that the junior name A. fundyense should be applied to group I. In contrast, Fraga et al. (2015) maintained that the oldest name A. catenella has priority and should be used. The proposal to use the name fundyense was rejected by the Nomenclature Committee, and if the two taxa are considered to belong to the same species, then the oldest name A. catenella must be used (Prud'homme van Reine 2017). The names Gonyaulax tamarensis var. excavata, Gonyaulax excavata and Alexandrium excavatum have been described from samples from the area of distribution of group I such as Norway and Argentina so it has to considered as a synonym of A. catenella [details] Introduced species impact Australian part of the Indian Ocean (Marine Region) Human health [details] Introduced species impact Chinese part of the South China Sea (Marine Region) Other impact - undefined or uncertain (Bloom forming) [details] Introduced species impact The recent expansion to other sites of the Sardinian coasts (A. Lugliè, pers. com.) and Ionian Sicilian coasts (M.G. Giacobbe, pers, com.), along with its toxicity and potential impact on human health, support that the species is invasive [details] Introduced species vector dispersal Chinese part of the South China Sea (Marine Region) Ships: General [details] Verified sequences Strain AL52: ITS/5.8S/ITS2/LSU rDNA KF646369 KF646375 KF646373 KF646372 (Wang et al. 2014) SSU rDNA JN098309 JN098323 JN626273 (Miranda et al. 2012) [details]
Published in AlgaeBase
 (from synonym Gonyaulax excavata (Braarud) Balech, 1971) (from synonym Gonyaulax excavata (Braarud) Balech, 1971)Published in AlgaeBase  (from synonym Gonyaulax tamarensis var. excavata T.Braarud, 1945) (from synonym Gonyaulax tamarensis var. excavata T.Braarud, 1945)Published in AlgaeBase  (from synonym Alexandrium fundyense Balech, 1985) (from synonym Alexandrium fundyense Balech, 1985)Published in AlgaeBase  Published in AlgaeBase  (from synonym Alexandrium acatenella (Whedon & Kofoid) Balech, 1985) (from synonym Alexandrium acatenella (Whedon & Kofoid) Balech, 1985)Published in AlgaeBase  (from synonym Gonyaulax acatenella Whedon & Kofoid, 1936) (from synonym Gonyaulax acatenella Whedon & Kofoid, 1936)Published in AlgaeBase  (from synonym Gonyaulax catenella Whedon & Kofoid, 1936) (from synonym Gonyaulax catenella Whedon & Kofoid, 1936)Published in AlgaeBase  (from synonym Gonyaulax washingtonensis Hsu, 1967) (from synonym Gonyaulax washingtonensis Hsu, 1967)Published in AlgaeBase  (from synonym Alexandrium excavatum (Braarud) Balech & Tangen, 1985) (from synonym Alexandrium excavatum (Braarud) Balech & Tangen, 1985)Published in AlgaeBase  (from synonym Protogonyaulax acatenella (Whedon & Kofoid) F.J.R.Taylor, 1979) (from synonym Protogonyaulax acatenella (Whedon & Kofoid) F.J.R.Taylor, 1979)Published in AlgaeBase  (from synonym Gessnerium acatenellum (Whedon & Kofoid) Loeblich & Loeblich III) (from synonym Gessnerium acatenellum (Whedon & Kofoid) Loeblich & Loeblich III)Published in AlgaeBase  (from synonym Protogonyaulax catenella (Whedon & Kofoid) Taylor, 1979) (from synonym Protogonyaulax catenella (Whedon & Kofoid) Taylor, 1979)Published in AlgaeBase  (from synonym Alexandrium tamarense f. excavatum (Braarud) Konovalova, 1993) (from synonym Alexandrium tamarense f. excavatum (Braarud) Konovalova, 1993)Published in AlgaeBase  (from synonym Protogonyaulax excavata (Braarud) F.J.R.Taylor, 1979) (from synonym Protogonyaulax excavata (Braarud) F.J.R.Taylor, 1979)Published in AlgaeBase  (from synonym Gessnerium catenella (Whedon & Kofoid) F.J.R.Taylor, 1979) (from synonym Gessnerium catenella (Whedon & Kofoid) F.J.R.Taylor, 1979)To Barcode of Life (10 barcodes) To Barcode of Life (3 barcodes) (from synonym Alexandrium fundyense Balech, 1985) To Biodiversity Heritage Library (1 publication) (from synonym Alexandrium acatenella (Whedon & Kofoid) Balech, 1985) To Biodiversity Heritage Library (1 publication) (from synonym Protogonyaulax acatenella (Whedon & Kofoid) F.J.R.Taylor, 1979) To Biodiversity Heritage Library (11 publications) (from synonym Gonyaulax excavata (Braarud) Balech, 1971) To Biodiversity Heritage Library (2 publications) (from synonym Gonyaulax tamarensis var. excavata T.Braarud, 1945) To Biodiversity Heritage Library (2 publications) (from synonym Protogonyaulax catenella (Whedon & Kofoid) Taylor, 1979) To Biodiversity Heritage Library (2 publications) (from synonym Alexandrium excavatum (Braarud) Balech & Tangen, 1985) To Biodiversity Heritage Library (27 publications) (from synonym Gonyaulax catenella Whedon & Kofoid, 1936) To Biodiversity Heritage Library (3 publications) (from synonym Gonyaulax acatenella Whedon & Kofoid, 1936) To Biodiversity Heritage Library (4 publications) (from synonym Alexandrium fundyense Balech, 1985) To Biodiversity Heritage Library (7 publications) To European Nucleotide Archive, ENA (Alexandrium catenella) To European Nucleotide Archive, ENA (Alexandrium excavatum) (from synonym Alexandrium excavatum (Braarud) Balech & Tangen, 1985) To European Nucleotide Archive, ENA (Alexandrium fundyense) (from synonym Alexandrium fundyense Balech, 1985) To GenBank (128971 nucleotides; 45689 proteins) To GenBank (3 nucleotides; 0 proteins) (from synonym Alexandrium excavatum (Braarud) Balech & Tangen, 1985) To GenBank (44147 nucleotides; 13420 proteins) (from synonym Alexandrium fundyense Balech, 1985) To PESI (from synonym Alexandrium fundyense Balech, 1985) To PESI (from synonym Gonyaulax tamarensis var. excavata T.Braarud, 1945) To PESI (from synonym Gonyaulax excavata (Braarud) Balech, 1971) To ITIS |