WoRMS taxon details
Lingulodinium polyedra (F.Stein) J.D.Dodge, 1989
233592 (urn:lsid:marinespecies.org:taxname:233592)
accepted
Species
Gonyaulax polyedra F.Stein, 1883 · unaccepted (synonym)
Hystrichosphaeridium machaerophorum Deflandre & Cookson, 1955 † · unaccepted (synonym)
marine, fresh, terrestrial
(of Gonyaulax polyedra F.Stein, 1883) Stein F.R.von. (1883). Der Organismus der Infusionstiere. III Abth. Der Organismus der Arthrodelen Flagellaten. <em>Einleitung und Erklarüng der Abbildungen.</em> II Hälfte: 23-26., available online at http://img.algaebase.org/pdf/1FBB00A00c42a348A7nLA99E031A/21711.pdf
page(s): 13, T4 [details]
page(s): 13, T4 [details]
Etymology Noun (Greek), Gr. poly, many; Gr. hedra, f. seat, chair, base, plane, side
LSID urn:lsid:algaebase.org:taxname:50026
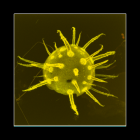
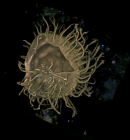
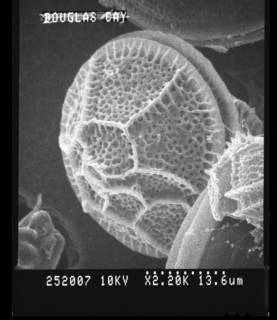
Description Cell size for all strains ranged from 37.6 to 52.5 μm in length and 33.8 to 47.9 μm in width. Mean size of strain K3-G8...
Etymology Noun (Greek), Gr. poly, many; Gr. hedra, f. seat, chair, base, plane, side [details]
LSID urn:lsid:algaebase.org:taxname:50026
LSID urn:lsid:algaebase.org:taxname:50026 [details]
Description Cell size for all strains ranged from 37.6 to 52.5 μm in length and 33.8 to 47.9 μm in width. Mean size of strain K3-G8...
Description Cell size for all strains ranged from 37.6 to 52.5 μm in length and 33.8 to 47.9 μm in width. Mean size of strain K3-G8 was 45.0 μm in length and 42.8 μm in width. Based on observations of swimming cells, there was no dorsoventral compression. Cells divided by desmochisis. Newly divided cells were identifiable by different degrees of ornamentation in maternal and new plates. The division line thereby determined, along which the maternal plates separated and being distributed to the daughter cells can be observed. The large nucleus had the shape of a hemi-torus and was located in the cingular plane, with both ends faintly visible in ventral focus of LM. The cells were brown-orange in colour with numerous small chloroplasts scattered throughout the cell. The cellsʼ outlines were heptagonal in ventral or dorsal view. The two obtuse angles of the in outline pentagonal epitheca resulted from the boundary between the precingular and apical plate series. The epitheca had a small, raised apical pore complex. The hypotheca was trapezoidal in outline with straight lines and a flat antapex without projections. In polar view, cells were nearly circular in outline. The cingulum was almost median, narrow (8–9% of cell length), incised and exhibited narrow cingular lists, which were ca 2.2 μm (range 1.8–2.8 μm, n = 20) in width. The cingulum was descending and displaced without overhang for ca two cingular widths. The plate formula was APC (Po, X, cp), 3′ , 3a, 6′ ′ , 6c, 6s, 6′ ′ ′ , 2′ ′ ′ ′. The epitheca comprised of six precingular plates, the APC and six climactal plates. Of these, three plates were in direct contact to the pore plate fulfilling the definition of apical plates. The ventrally located plate 1′ was narrow, irregular in shape and ranged from the anterior sulcal plate to the pore plate. Plate 2′ on the left-lateral side was heptagonal and large. Apical plate 3′ right of the pore plate was small and hexagonal. On the cells’ right side, there were three anterior intercalary plates, which were all pentagonal and approximately of the same size. Within the APC, there was an elongated oval pore plate, which had a few pores and which was bordered by a raised rim formed by the adjacent apical plates 2′ and 3′ . Ventrally, the pore plate abutted the first apical plate, which was difficult to observe, because the rim of plates 2′ and 3′ tended to overgrow plate 1′ in its anterior part. In the centre of the pore plate, there was a narrow tube with an oval outline and terminated by a cover plate, which was flecked by small, elongate structures. Ventrally, the pore plate had a more or less triangular notch, here a small X-plate was located. This X-plate abutted plate 1′ and continued from the base of the pore plate all along the rise of the apical pore to the cover plate. If the contact of precingular plates to the cingulum was considered a single side (i.e., irrespective whether one or two cingular plates were contacted), then plates 1′ ′ , 3′ ′ , 4′ ′ and 5′ ′ were pentagonal, whereas the left-lateral plate 2′ ′ was tetragonal. The precingular plate 6′ ′ was in contact with the sulcal plate sda and was thus hexagonal. A ventral pore was present at the right margin of plate 1′ , approximately in the middle of the suture between plates 1′ and 3a. The pore seemed to be always present, even if it was difficult to detect when, for example, out of focus or when partly hidden by the overlapping plate margin of plate 3a. This ventral pore was made by a platelike tube located in a circular notch of plate 1′. The mean diameter of the tube was 0.71±0.08 μm (0.58–0.84 μm, n = 10). There were six cingular plates all being of almost the same size. The sulcus was a narrow and incised concave groove bordered by sulcal lists, which widened posteriorly and reached the antapex. Its anterior part was mostly hidden due to a close rank of plate 1′ ′ ′ on the left and plate sda on the right. With its anterior part, plate sa slightly invaded the epitheca. Pl [details]
Guiry, M.D. & Guiry, G.M. (2024). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway (taxonomic information republished from AlgaeBase with permission of M.D. Guiry). Lingulodinium polyedra (F.Stein) J.D.Dodge, 1989. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=233592 on 2024-04-26
Date
action
by
2006-07-27 06:59:07Z
created
Camba Reu, Cibran
Copyright notice: the information originating from AlgaeBase may not be downloaded or replicated by any means, without the written permission of the copyright owner (generally AlgaeBase). Fair usage of data in scientific publications is permitted.
original description
(of Gonyaulax polyedra F.Stein, 1883) Stein F.R.von. (1883). Der Organismus der Infusionstiere. III Abth. Der Organismus der Arthrodelen Flagellaten. <em>Einleitung und Erklarüng der Abbildungen.</em> II Hälfte: 23-26., available online at http://img.algaebase.org/pdf/1FBB00A00c42a348A7nLA99E031A/21711.pdf
page(s): 13, T4 [details]
context source (HKRMS) Clark, A. M. (1982). Echinoderms of Hong Kong. <em>In: Morton B, editor. Proceedings of the first international marine biological workshop: The marine flora and fauna of Hong Kong and southern China. Hong Kong University Press, Hong Kong.</em> 1: 485-501. [details]
basis of record Gómez, F. (2005). A list of free-living dinoflagellate species in the world's oceans. <em>Acta Bot. Croat.</em> 64(1): 129-212. [details]
additional source Zenetos, A.; Çinar, M.E.; Pancucci-Papadopoulou, M.A.; Harmelin, J.-G.; Furnari, G.; Andaloro, F.; Bellou, N.; Streftaris, N.; Zibrowius, H. (2005). Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. <em>Mediterranean Marine Science.</em> 6 (2): 63-118., available online at https://www.researchgate.net/publication/273213810_Annotated_list_of_marine_alien_species_in_the_Mediterranean_with_records_of_the_worst_invasive_species [details] Available for editors [request]
[request]
additional source Steidinger, K. A., M. A. Faust, and D. U. Hernández-Becerril. 2009. Dinoflagellates (Dinoflagellata) of the Gulf of Mexico, Pp. 131–154 in Felder, D.L. and D.K. Camp (eds.), Gulf of Mexico–Origins, Waters, and Biota. Biodiversity. Texas A&M Press, College [details]
additional source Guiry, M.D. & Guiry, G.M. (2023). AlgaeBase. <em>World-wide electronic publication, National University of Ireland, Galway.</em> searched on YYYY-MM-DD., available online at http://www.algaebase.org [details]
additional source Tomas, C.R. (Ed.). (1997). Identifying marine phytoplankton. Academic Press: San Diego, CA [etc.] (USA). ISBN 0-12-693018-X. XV, 858 pp., available online at http://www.sciencedirect.com/science/book/9780126930184 [details]
additional source Moestrup, Ø., Akselman, R., Cronberg, G., Elbraechter, M., Fraga, S., Halim, Y., Hansen, G., Hoppenrath, M., Larsen, J., Lundholm, N., Nguyen, L. N., Zingone, A. (Eds) (2009 onwards). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae., available online at http://www.marinespecies.org/HAB [details]
additional source Chang, F.H.; Charleston, W.A.G.; McKenna, P.B.; Clowes, C.D.; Wilson, G.J.; Broady, P.A. (2012). Phylum Myzozoa: dinoflagellates, perkinsids, ellobiopsids, sporozoans, in: Gordon, D.P. (Ed.) (2012). New Zealand inventory of biodiversity: 3. Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi. pp. 175-216. [details]
new combination reference Dodge, J.D. 1989. Some Revisions of the Family Gonyaulacaceae (Dinophyceae) Based on Scanning Electron Microscope Study. Bot. Mar. 32:275-298 [details] Available for editors [request]
[request]
toxicology source Paz, B., Riobó, P., Fernández, M. L., Fraga, S. & Franco, J. M. 2004. Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon 44:251-58. [details] Available for editors [request]
[request]
ecology source Jeong, H.; Yoo, Y.; Park, J.; Song, J.; Kim, S.; Lee, S.; Kim, K.; Yih, W. (2005). Feeding by phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. <em>Aquatic Microbial Ecology.</em> 40: 133-150., available online at https://doi.org/10.3354/ame040133 [details]
ecology source Leles, S. G.; Mitra, A.; Flynn, K. J.; Tillmann, U.; Stoecker, D.; Jeong, H. J.; Burkholder, J.; Hansen, P. J.; Caron, D. A.; Glibert, P. M.; Hallegraeff, G.; Raven, J. A.; Sanders, R. W.; Zubkov, M. (2019). Sampling bias misrepresents the biogeographical significance of constitutive mixotrophs across global oceans. <em>Global Ecology and Biogeography.</em> 28(4): 418-428., available online at https://doi.org/10.1111/geb.12853 [details] Available for editors [request]
[request]
ecology source Mitra, A.; Caron, D. A.; Faure, E.; Flynn, K. J.; Leles, S. G.; Hansen, P. J.; McManus, G. B.; Not, F.; Do Rosario Gomes, H.; Santoferrara, L. F.; Stoecker, D. K.; Tillmann, U. (2023). The Mixoplankton Database (MDB): Diversity of photo‐phago‐trophic plankton in form, function, and distribution across the global ocean. <em>Journal of Eukaryotic Microbiology.</em> 70(4)., available online at https://doi.org/10.1111/jeu.12972 [details]
ecology source Jeong, H.; Park, J.; Nho, J.; Park, M.; Ha, J.; Seong, K.; Jeng, C.; Seong, C.; Lee, K.; Yih, W. (2005). Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. <em>Aquatic Microbial Ecology.</em> 41: 131-143., available online at https://doi.org/10.3354/ame041131 [details]
ecology source Du Yoo, Y.; Jeong, H. J.; Kim, M. S.; Kang, N. S.; Song, J. Y.; Shin, W.; Kim, K. Y.; Lee, K. (2009). Feeding by Phototrophic Red-Tide Dinoflagellates on the Ubiquitous Marine Diatom<i>Skeletonema costatum</i>. <em>Journal of Eukaryotic Microbiology.</em> 56(5): 413-420., available online at https://doi.org/10.1111/j.1550-7408.2009.00421.x [details]
page(s): 13, T4 [details]
context source (HKRMS) Clark, A. M. (1982). Echinoderms of Hong Kong. <em>In: Morton B, editor. Proceedings of the first international marine biological workshop: The marine flora and fauna of Hong Kong and southern China. Hong Kong University Press, Hong Kong.</em> 1: 485-501. [details]
basis of record Gómez, F. (2005). A list of free-living dinoflagellate species in the world's oceans. <em>Acta Bot. Croat.</em> 64(1): 129-212. [details]
additional source Zenetos, A.; Çinar, M.E.; Pancucci-Papadopoulou, M.A.; Harmelin, J.-G.; Furnari, G.; Andaloro, F.; Bellou, N.; Streftaris, N.; Zibrowius, H. (2005). Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. <em>Mediterranean Marine Science.</em> 6 (2): 63-118., available online at https://www.researchgate.net/publication/273213810_Annotated_list_of_marine_alien_species_in_the_Mediterranean_with_records_of_the_worst_invasive_species [details] Available for editors
additional source Steidinger, K. A., M. A. Faust, and D. U. Hernández-Becerril. 2009. Dinoflagellates (Dinoflagellata) of the Gulf of Mexico, Pp. 131–154 in Felder, D.L. and D.K. Camp (eds.), Gulf of Mexico–Origins, Waters, and Biota. Biodiversity. Texas A&M Press, College [details]
additional source Guiry, M.D. & Guiry, G.M. (2023). AlgaeBase. <em>World-wide electronic publication, National University of Ireland, Galway.</em> searched on YYYY-MM-DD., available online at http://www.algaebase.org [details]
additional source Tomas, C.R. (Ed.). (1997). Identifying marine phytoplankton. Academic Press: San Diego, CA [etc.] (USA). ISBN 0-12-693018-X. XV, 858 pp., available online at http://www.sciencedirect.com/science/book/9780126930184 [details]
additional source Moestrup, Ø., Akselman, R., Cronberg, G., Elbraechter, M., Fraga, S., Halim, Y., Hansen, G., Hoppenrath, M., Larsen, J., Lundholm, N., Nguyen, L. N., Zingone, A. (Eds) (2009 onwards). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae., available online at http://www.marinespecies.org/HAB [details]
additional source Chang, F.H.; Charleston, W.A.G.; McKenna, P.B.; Clowes, C.D.; Wilson, G.J.; Broady, P.A. (2012). Phylum Myzozoa: dinoflagellates, perkinsids, ellobiopsids, sporozoans, in: Gordon, D.P. (Ed.) (2012). New Zealand inventory of biodiversity: 3. Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi. pp. 175-216. [details]
new combination reference Dodge, J.D. 1989. Some Revisions of the Family Gonyaulacaceae (Dinophyceae) Based on Scanning Electron Microscope Study. Bot. Mar. 32:275-298 [details] Available for editors
toxicology source Paz, B., Riobó, P., Fernández, M. L., Fraga, S. & Franco, J. M. 2004. Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon 44:251-58. [details] Available for editors
ecology source Jeong, H.; Yoo, Y.; Park, J.; Song, J.; Kim, S.; Lee, S.; Kim, K.; Yih, W. (2005). Feeding by phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. <em>Aquatic Microbial Ecology.</em> 40: 133-150., available online at https://doi.org/10.3354/ame040133 [details]
ecology source Leles, S. G.; Mitra, A.; Flynn, K. J.; Tillmann, U.; Stoecker, D.; Jeong, H. J.; Burkholder, J.; Hansen, P. J.; Caron, D. A.; Glibert, P. M.; Hallegraeff, G.; Raven, J. A.; Sanders, R. W.; Zubkov, M. (2019). Sampling bias misrepresents the biogeographical significance of constitutive mixotrophs across global oceans. <em>Global Ecology and Biogeography.</em> 28(4): 418-428., available online at https://doi.org/10.1111/geb.12853 [details] Available for editors
ecology source Mitra, A.; Caron, D. A.; Faure, E.; Flynn, K. J.; Leles, S. G.; Hansen, P. J.; McManus, G. B.; Not, F.; Do Rosario Gomes, H.; Santoferrara, L. F.; Stoecker, D. K.; Tillmann, U. (2023). The Mixoplankton Database (MDB): Diversity of photo‐phago‐trophic plankton in form, function, and distribution across the global ocean. <em>Journal of Eukaryotic Microbiology.</em> 70(4)., available online at https://doi.org/10.1111/jeu.12972 [details]
ecology source Jeong, H.; Park, J.; Nho, J.; Park, M.; Ha, J.; Seong, K.; Jeng, C.; Seong, C.; Lee, K.; Yih, W. (2005). Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. <em>Aquatic Microbial Ecology.</em> 41: 131-143., available online at https://doi.org/10.3354/ame041131 [details]
ecology source Du Yoo, Y.; Jeong, H. J.; Kim, M. S.; Kang, N. S.; Song, J. Y.; Shin, W.; Kim, K. Y.; Lee, K. (2009). Feeding by Phototrophic Red-Tide Dinoflagellates on the Ubiquitous Marine Diatom<i>Skeletonema costatum</i>. <em>Journal of Eukaryotic Microbiology.</em> 56(5): 413-420., available online at https://doi.org/10.1111/j.1550-7408.2009.00421.x [details]
 Present
Present  Present in aphia/obis/gbif/idigbio
Present in aphia/obis/gbif/idigbio  Inaccurate
Inaccurate  Introduced: alien
Introduced: alien  Containing type locality
Containing type locality
From editor or global species database
Etymology Noun (Greek), Gr. poly, many; Gr. hedra, f. seat, chair, base, plane, side [details]LSID urn:lsid:algaebase.org:taxname:50026 [details]
Spelling The epithet "polyedra" is a noun in apposition and is non-declinable, so use of the epithet "polyedrum", supposedly to agree with the gender of the genus name, is incorrect. ICN Art 23.5 [Melbourne Code]: "The specific epithet, when adjectival in form and not used as a noun, agrees grammatically with the generic name; when it is a noun in apposition or a genitive noun, it retains its own gender and termination irrespective of the gender of the generic name. Epithets not conforming to this rule are to be corrected (see Art. 32.2) [details]
From regional or thematic species database
Description Cell size for all strains ranged from 37.6 to 52.5 μm in length and 33.8 to 47.9 μm in width. Mean size of strain K3-G8 was 45.0 μm in length and 42.8 μm in width. Based on observations of swimming cells, there was no dorsoventral compression. Cells divided by desmochisis. Newly divided cells were identifiable by different degrees of ornamentation in maternal and new plates. The division line thereby determined, along which the maternal plates separated and being distributed to the daughter cells can be observed. The large nucleus had the shape of a hemi-torus and was located in the cingular plane, with both ends faintly visible in ventral focus of LM. The cells were brown-orange in colour with numerous small chloroplasts scattered throughout the cell. The cellsʼ outlines were heptagonal in ventral or dorsal view. The two obtuse angles of the in outline pentagonal epitheca resulted from the boundary between the precingular and apical plate series. The epitheca had a small, raised apical pore complex. The hypotheca was trapezoidal in outline with straight lines and a flat antapex without projections. In polar view, cells were nearly circular in outline. The cingulum was almost median, narrow (8–9% of cell length), incised and exhibited narrow cingular lists, which were ca 2.2 μm (range 1.8–2.8 μm, n = 20) in width. The cingulum was descending and displaced without overhang for ca two cingular widths. The plate formula was APC (Po, X, cp), 3′ , 3a, 6′ ′ , 6c, 6s, 6′ ′ ′ , 2′ ′ ′ ′. The epitheca comprised of six precingular plates, the APC and six climactal plates. Of these, three plates were in direct contact to the pore plate fulfilling the definition of apical plates. The ventrally located plate 1′ was narrow, irregular in shape and ranged from the anterior sulcal plate to the pore plate. Plate 2′ on the left-lateral side was heptagonal and large. Apical plate 3′ right of the pore plate was small and hexagonal. On the cells’ right side, there were three anterior intercalary plates, which were all pentagonal and approximately of the same size. Within the APC, there was an elongated oval pore plate, which had a few pores and which was bordered by a raised rim formed by the adjacent apical plates 2′ and 3′ . Ventrally, the pore plate abutted the first apical plate, which was difficult to observe, because the rim of plates 2′ and 3′ tended to overgrow plate 1′ in its anterior part. In the centre of the pore plate, there was a narrow tube with an oval outline and terminated by a cover plate, which was flecked by small, elongate structures. Ventrally, the pore plate had a more or less triangular notch, here a small X-plate was located. This X-plate abutted plate 1′ and continued from the base of the pore plate all along the rise of the apical pore to the cover plate. If the contact of precingular plates to the cingulum was considered a single side (i.e., irrespective whether one or two cingular plates were contacted), then plates 1′ ′ , 3′ ′ , 4′ ′ and 5′ ′ were pentagonal, whereas the left-lateral plate 2′ ′ was tetragonal. The precingular plate 6′ ′ was in contact with the sulcal plate sda and was thus hexagonal. A ventral pore was present at the right margin of plate 1′ , approximately in the middle of the suture between plates 1′ and 3a. The pore seemed to be always present, even if it was difficult to detect when, for example, out of focus or when partly hidden by the overlapping plate margin of plate 3a. This ventral pore was made by a platelike tube located in a circular notch of plate 1′. The mean diameter of the tube was 0.71±0.08 μm (0.58–0.84 μm, n = 10). There were six cingular plates all being of almost the same size. The sulcus was a narrow and incised concave groove bordered by sulcal lists, which widened posteriorly and reached the antapex. Its anterior part was mostly hidden due to a close rank of plate 1′ ′ ′ on the left and plate sda on the right. With its anterior part, plate sa slightly invaded the epitheca. Pl [details]Harmful effect Paralyzing toxins are potentially also produced by this dinoflagellate, as some authors have found saxitoxin (or ‘STX-like substances’) in L. polyedra extracts obtained from a red tide sample collected in Italy in 1988 (Bates et al., 1978; Bruno et al., 1990). It is known that Lingulodinium polyedra produces YTX and probably homo-YTX, as confirmed by the analysis of Spanish cultures and analysis of a sample from the Adriatic (Yasumoto and Satake, 1998; Paz et al, 2004). In L. polyedra cultures, YTX has been detected in both the cell fraction and in the culture medium (Paz et al., 2004). [details]
Identification A closely related species is Lingulodinium milneri. From Dodge 1989.
[details]
Published in AlgaeBase  (from synonym Gonyaulax polyedra F.Stein, 1883)
(from synonym Gonyaulax polyedra F.Stein, 1883)
Published in AlgaeBase
Published in AlgaeBase (from synonym Lingulodinium machaerophorum (Deflandre & Cookson) D.Wall, 1967 †)
(from synonym Lingulodinium machaerophorum (Deflandre & Cookson) D.Wall, 1967 †)
Published in AlgaeBase (from synonym Hystrichosphaeridium machaerophorum Deflandre & Cookson, 1955 †)
(from synonym Hystrichosphaeridium machaerophorum Deflandre & Cookson, 1955 †)
To Biodiversity Heritage Library (4 publications) (from synonym Hystrichosphaeridium machaerophorum Deflandre & Cookson, 1955 †)
To Biodiversity Heritage Library (5 publications) (from synonym Lingulodinium machaerophorum (Deflandre & Cookson) D.Wall, 1967 †)
To Biodiversity Heritage Library (6 publications)
To Biodiversity Heritage Library (89 publications) (from synonym Gonyaulax polyedra F.Stein, 1883)
To Dyntaxa
To European Nucleotide Archive (ENA)
To GenBank (337078 nucleotides; 62439 proteins)
To GenBank (337078 nucleotides; 62439 proteins) (from synonym Gonyaulax polyedra F.Stein, 1883)
To NMNH Extant Collection (252007.jpg) (from synonym Gonyaulax polyedra F.Stein, 1883)
To PESI (from synonym Gonyaulax polyedra F.Stein, 1883)
To PESI
To ITIS
 (from synonym Gonyaulax polyedra F.Stein, 1883)
(from synonym Gonyaulax polyedra F.Stein, 1883)Published in AlgaeBase
Published in AlgaeBase
 (from synonym Lingulodinium machaerophorum (Deflandre & Cookson) D.Wall, 1967 †)
(from synonym Lingulodinium machaerophorum (Deflandre & Cookson) D.Wall, 1967 †)Published in AlgaeBase
 (from synonym Hystrichosphaeridium machaerophorum Deflandre & Cookson, 1955 †)
(from synonym Hystrichosphaeridium machaerophorum Deflandre & Cookson, 1955 †)To Biodiversity Heritage Library (4 publications) (from synonym Hystrichosphaeridium machaerophorum Deflandre & Cookson, 1955 †)
To Biodiversity Heritage Library (5 publications) (from synonym Lingulodinium machaerophorum (Deflandre & Cookson) D.Wall, 1967 †)
To Biodiversity Heritage Library (6 publications)
To Biodiversity Heritage Library (89 publications) (from synonym Gonyaulax polyedra F.Stein, 1883)
To Dyntaxa
To European Nucleotide Archive (ENA)
To GenBank (337078 nucleotides; 62439 proteins)
To GenBank (337078 nucleotides; 62439 proteins) (from synonym Gonyaulax polyedra F.Stein, 1883)
To NMNH Extant Collection (252007.jpg) (from synonym Gonyaulax polyedra F.Stein, 1883)
To PESI (from synonym Gonyaulax polyedra F.Stein, 1883)
To PESI
To ITIS